Last updated: November 3, 2021
Vaccinating Kids 5 and Up
Parents or legal guardians of minors must physically accompany the minor to the vaccination appointment and provide consents for care.
Schedule for your child today
COVID-19 Booster Updates
The CDC has approved Moderna and J&J COVID-19 vaccine boosters and cleared the way to switch brands when getting a booster.
More Booster Information
View information on Emergency Use Authorization (EUA) and vaccine consent documents.
COVID-19 Vaccination Program | Scheduling is Fast and Easy | What to Know Before You Schedule | What to Know Before You Go to Your Appointment | Vaccine FAQs | Additional Vaccine Options | NorthShore Virtual Townhall Meeting | Why I'm Vaccinating | COVID Community Checkup | Vaccine News | Social Media Posts
COVID-19 Vaccination Program
- We are now vaccinating all individuals 5 and older and administering booster vaccinations to eligible cohorts.
- Note that children 5-11 will receive less dosage than teens and adults (two 10 microgram doses compared to two 30 microgram doses given to individuals 12 and up.)
- NorthShore and Swedish facilities are now accepting appointments (and, at Swedish Hospital, walk-ins) for COVID-19 vaccination.
- Parents or legal guardians of minors (i.e. all patients under 18 years-old) must physically accompany the minor to the vaccination appointment and provide consents for care for vaccine to be administered.
Scheduling is Fast and Easy
- Self-schedule via your NorthShoreConnect patient portal. Don't have an account? Sign up today to schedule your vaccine appointment, to get easy future access to your vaccine records and more!
- For additional assistance in scheduling, you may call 847.982.5000 (note wait times may be longer than normal due to higher call volumes).
- Swedish Hospital is also accepting walk-ins
What to Know Before You Schedule
- Parents or guardians of individuals (all those under 18 years-old) must physically accompany the minor to the scheduled appointment and provide consents for care and vaccination. Vaccines cannot be provided to minors without onsite parental or legal guardian authorization.
- Your second dose will be automatically scheduled for the same time, date and location 21 (Pfizer) or 28 (Moderna) days following your first dose.
- You are not fully vaccinated until 14 days after your second dose. Please commit to come to your second dose appointment specially reserved for you.
- The CDC has lifted its original imposed grace period between receiving COVID-19 vaccines and other vaccines. COVID-19 vaccines and other vaccines may now be administered without regard to timing and may even be co-administered.
- Please review the Emergency Use Authorization for COVID-19 vaccines documents.
What to Know Before You Go to Your Appointment
- If you are experiencing COVID-19 like symptoms or have had a high-risk exposure and have not met the quarantine and recovery criteria period, please cancel your appointment
- If you have an active COVID-19 infection, you may receive the COVID-19 vaccine following 10-days of isolation and the resolution of symptoms (including being fever-free for 24 hours).
- If you have had a high risk COVID-19 exposure (defined as: either yourself or the person confirmed with COVID-19 infection were unmasked, within six feet, and you were exposed greater or equal to a total of 15 minutes over a period of 24 hours), you may receive the COVID-19 vaccine following 14-days of quarantine from the exposure.
Vaccine FAQs
Is one COVID vaccine better than another?
No. All vaccines are approved by the FDA are safe and effective against the SARS Co-V 2 virus (COVID 19). At this time NorthShore is administering the Pfizer vaccine per the FDA and CDC recommendations.
Will I be charged for my COVID-19 vaccination?
There’s no charge for COVID-19 vaccinations or their administration. Those without health insurance can get vaccinated without out-of-pocket costs. If you believe you have been charged for your vaccine, please contact our Billing Customer Service office at 847.570.5000.
Can I get COVID-19 from the vaccine?
No. While the vaccine doesn’t contain either live or dead virus, you may develop “flu-like” symptoms such as tiredness, fevers or headaches after receiving one or both doses of the vaccine but these usually resolve within three days.
If someone has previously had COVID-19, should they get the vaccine?
Due to the severe health risks associated with COVID-19 and the fact that re-infection is possible, people may be advised to get a vaccine even if they have been sick with the virus before. If you have had a previous COVID infection, you should wait at least 10 days since your symptom-onset or test date with resolving symptoms before receiving the vaccine.
I’ve heard the vaccine may cause infertility. Is that true?
This is a myth. There’s no data to suggest the COVID vaccine causes infertility. There also has been no changes to fertility rates in people who’ve had COVID and have recovered. A few other myths about the vaccine also are unfounded: The vaccine does not insert a “chip” or permanent marker in your genetic material. Both vaccines are mRNA and will naturally degrade over time like other mRNA your body makes. And the vaccine can’t give you COVID.
Vaccine FAQs for Minors
Is the vaccine safe for kids?
Pfizer’s trial originally included 2,268 children and then subsequently expanded it to 4,500 children. Two-thirds received two doses of the vaccine three weeks apart; one-third received two injections of a saline placebo. According to Pfizer’s data for this group, the vaccine was 90.7% effective in preventing symptomatic COVID-19. Kids who received the two-dose regimen experienced a robust antibody response similar to that experience by older kids. The study found the Pfizer vaccine to be safe, effective and well-tolerated with no cases of severe illness or heart conditions. Mild side effects were on par with those experienced by older kids and last one-to-three days.
The FDA evaluated the safety and efficacy of Pfizer’s vaccine for younger children, saying that the benefits of preventing severe cases of COVID-19 with the Pfizer vaccine generally outweighed the risks.
Is the vaccine dosage for younger kids different than for teens and adults?
Yes. Children in Pfizer’s 5-to-11-year-old group received two 10-microgram doses. By comparison, older kids (age 12-17) received two 30-microgram doses. Trial studies found that a fractional dosage produced an immune response in younger kids that was roughly equivalent to the immune response experienced in patients 16-to-25 years old. This may be because that younger kids have more robust immune systems.
Are the vaccine side effects different than for teens and adults?
The side effects for children from clinical trials are on par with those experienced by adolescents. The FDA reports the most commonly reported side effects in the adolescent trial were pain at the injection site, fatigue, headache, chills, muscle pain, fever, and joint pain. Side effects faded quickly, typically in one-to-three days and can be treated with rest and over-the-counter remedies.
Is it safe to give young kids a new vaccine?
Hundreds of millions of people in the U.S. have received COVID-19 vaccines in the largest and most scrutinized vaccine program in the country’s history. Among them: millions of kids 12 and up have shown the vaccine to be safe and effective in younger patients.
While COVID-19 vaccines are new, the mRNA technology used in Pfizer’s and Moderna’s vaccines has been studied for about 15 years.
mRNA molecules occur naturally in humans, and the molecules from the vaccine are destroyed and don’t stay in the body. This means the vaccines are safe for kids’ growing bodies and don’t affect puberty, fertility, or brain development.
COVID is less common and less dangerous in kids, so do they really need to get vaccinated?
Yes. COVID-19 is safe and effective and protects the children themselves as well as older and more vulnerable people with whom they interact—grandparents, parents, teachers, etc. Their chance to get vaccinated and stay healthy comes ahead of the return to winter—when cold weather drives many kids’ activities indoors and COVID-19 cases and variants tend to surge.
My child has severe allergies. Is it safe for them to receive a COVID-19 vaccine?
In general, it is safe for a child with severe food, venom, or medication allergies to receive COVID-19 vaccine. Currently, the COVID-19 vaccine approved for emergency use in 5-year-olds and older is the Pfizer vaccine. Children allergic to any component of the COVID-19 vaccine should not receive the vaccine.
In addition, NorthShore University HealthSystem is conducting a National Institutes of Health (NIH)-sponsored clinical trial investigating how patients with histories of severe allergies react to the Pfizer and Moderna COVID-19 vaccines. This trial is open to participants age 12 and older. Participants will be observed for 90 minutes by an allergist after vaccine administration. If you are interested in participating in this clinical trial, please reach out to your allergist or email ctc@northshore.org.
Johnson & Johnson Vaccine Information
Is NorthShore administering Johnson & Johnson?
NorthShore currently is providing only the Pfizer vaccine. While Johnson & Johnson has been newly approved for general use, NorthShore has not been provided and therefore is not administering Johnson & Johnson vaccines at this time.
Vaccine Information for Cancer/Immunocompromised Patients
Additional guidelines are provided and referenced from The American Society of Clinical Oncology (ASCO) and The National Cancer Care Network (NCCN).
Will my chemotherapy or other medication which affects my immune system decrease my response to the COVID-19 vaccine? What about for bone marrow transplant patients?
Because any vaccine relies on the immune system to create the protection against the infection, it is possible that people who are immunocompromised or have a weakened immune system may not have as good of a response to the COVID-19 vaccine. However, in the vaccine administered groups, participants who developed COVID-19 infection had much milder disease. Thus, it is possible that if you receive the vaccine, you may not be fully protected from infection; however, if you develop an infection it is likely to be much milder than without the vaccine. It is not specifically known if patients receiving/who have received a bone marrow transplant would have a different response, and this should be discussed with your transplant physician.
Will the COVID-19 vaccine cause any harmful effects on my cancer?
This is unknown, but experience with many other vaccines indicates this is quite unlikely. However, because there are significant risks to cancer patients with COVID-19 infection there is likely to be more benefit from the vaccine than any risk to making the cancer worse.
Potential vaccine side effects and allergic reactions
What insights can you provide on any potential vaccine side effects for various populations—including the elderly and younger children?
We currently only have safety data from the short-term duration of the study trial. This means that we don’t know all of the potential side effects of the vaccine, particularly long-term side effects. We do know that the following have been reported as possible side effects which can last up to a week after receiving the vaccine:
- Pain and redness at the injection site
- Feeling tired and run down a day after receiving the vaccine
- Headache, muscle aches, chills, joint pains, or fever
- Possible allergic reaction in individuals with known allergies
These symptoms are a sign that your immune system is mounting a response to the vaccine which is good, and is to be expected in an immune response. Remember: these symptoms are expected side effects and aren’t allergies. You should complete the two-dose vaccination series.
What do we know about allergies to vaccines, and the COVID-19 vaccine in particular?
Anaphylaxis after vaccination can be seen with any vaccine or medication. However, it’s rare and often occurs in patients with no prior history of allergic reaction. The risk for anaphylaxis to vaccines has been reported to be approximately one in every million doses. To put this in perspective, the risk of anaphylaxis to penicillin is much higher—approximately one out of 2,000-10,000 courses of treatment.
What precautions are recommended?
Because rare cases of anaphylaxis to vaccines happen, it’s recommended that vaccines be administered in settings equipped with epinephrine and medical personnel trained to treat allergic reactions. With 21 million doses of the Pfizer vaccine expected to be distributed in the coming weeks, we may see some allergic reactions. That’s why a 15-minute post-observation period is recommended for everyone who is vaccinated at this time. Anyone with a history of anaphylaxis, the observation period will be extended to 30 minutes. Please keep in mind that this a new vaccine and a constantly evolving situation.
What if I have a reaction that isn’t anaphylaxis? Could it be an allergy to the vaccine itself?
If you experience hives or itching, shortness of breath, runny nose, congestion, tongue or throat discomfort, these symptoms need to be further discussed with an allergist to determine the best next steps. If you experience any of these type of symptoms, we’ll refer you to NorthShore allergists so you can follow up within 7 days so any additional guidance regarding administration of the second dose of COVID-19 vaccine can be incorporated into your care.
Additional Vaccine Options
We encourage you to sign up at all area locations that offer vaccination to increase your chances of getting vaccinated as soon as possible.
Please visit the Illinois Department of Public Health website for more information.
NorthShore Virtual Town Hall Meeting
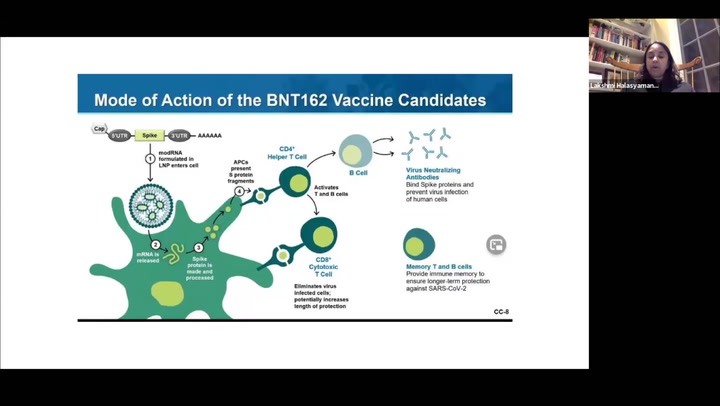
On Tuesday, February 16th, NorthShore University HealthSystem held a virtual town hall meeting. Lakshmi Halasyamani, MD, Chief Medical Officer shared her information about how the COVID-19 vaccination works, statistics on how Illinois’s rollout is going and a Q and A session with questions from the community. Watch the archived live stream below.
Testimonials